Q4 BMF meeting looks at automation in life sciences manufacturing
Current automation trends in the life sciences was the topic of discussion at the fourth quarter meeting of the NCLifeSci Biotech Manufacturers Forum held Dec. 2 at the NC Biotechnology Center. The meeting featured presentations by experts from the Advanced Technology Group, GXP-Storage and Kymanox.
NCLifeSci President Laura Gunter provided an overview of state and federal legislative activities. BMF Program Manager Bill Monteith welcomed SAS as a new member of the forum and he and Jenae Williams, NCLifeSci workforce and partnership director, gave updates on BMF events and initiatives.
After the business meeting concluded, attention turned to presentations by
- Jason Merryman, senior director of manufacturing and automation engineering, Kymanox
- Oliver Bausch, president/owner, Advanced Technology Group
- Patrick Steffens, director of IT, GXP-Storage
Achieving automation success in life sciences
Merryman shared his insights on how companies can choose the right automation partners and navigate the journey from planning to execution.
Merryman emphasized the importance of clearly defining automation objectives from the outset.
"In most cases, the primary strategy for automation in life sciences is to improve patient safety," he said. "Other benefits like cost savings and capacity expansion are important, but secondary."
Before embarking on an automation initiative, Merryman advised companies to optimize their existing processes. Drawing on Elon Musk's engineering principles, he recommended verifying and eliminating unnecessary requirements, deleting redundant process steps, and simplifying workflows.
"Why optimize something that shouldn't exist in the first place?" he asked.
Merryman also stressed the importance of automating critical quality control steps and accommodating risk.
Overcoming barriers is a key challenge in automation adoption. Merryman identified cost, complexity, workforce concerns and regulatory hurdles as major obstacles. To address these, he suggested choosing the right automation partner, engaging experienced commissioning and validation experts and continuously communicating the strategic rationale to employees.
"Hire your technical resources early and train them as early as possible," he advised.
When it comes to navigating the planning and execution phases, Merryman emphasized the need for a risk-based mindset.
"Plan for everything," he said, highlighting the importance of thorough risk assessments, process maps and validation plans. During the execution phase, he stressed the value of having technical personnel embedded on the production floor to ensure a smooth transition.
Looking ahead, Merryman discussed several emerging trends in life science automation, including the rise of integrated manufacturing execution systems, flexible automation using robots and the increasing adoption of process analytical technology. He also highlighted the importance of risk-based maintenance programs to optimize asset performance and minimize downtime.
Automating sterile gene and cell therapy operations
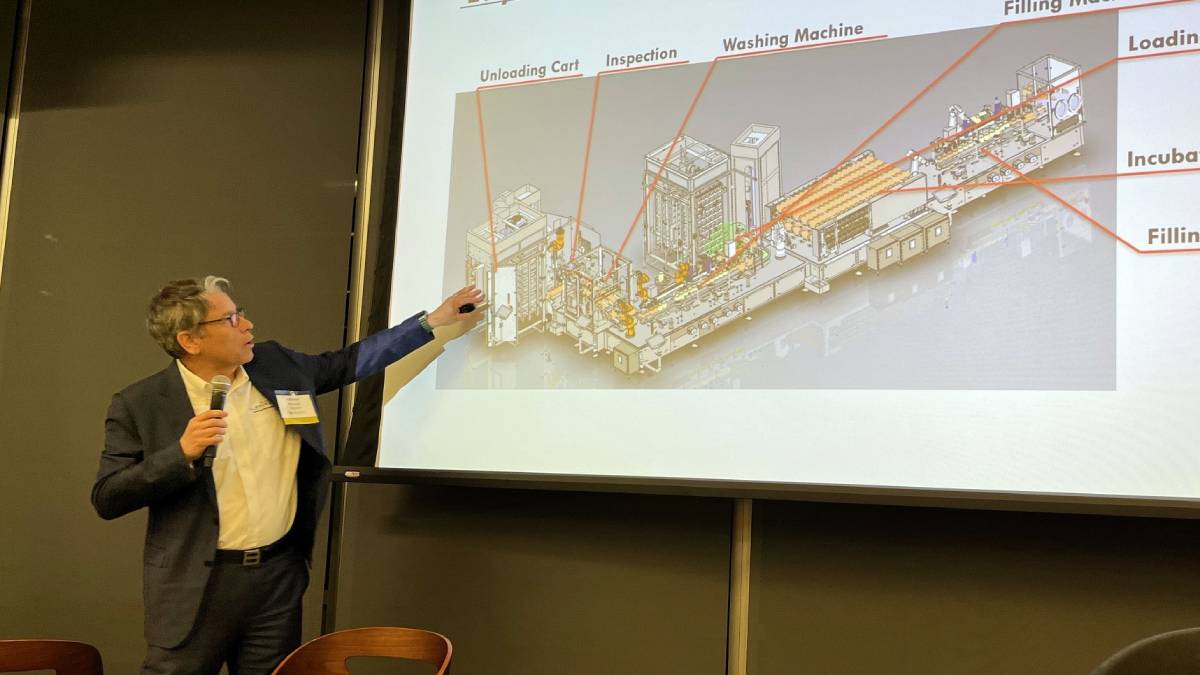
Bausch provided a detailed look at a fully automated production line his company recently delivered to a customer in sterile gene and cell therapy manufacturing.
Bausch began by highlighting the key benefits of automation, including improved quality and higher output rates. The automated line he described is capable of scaling up production from a single liter per minute to 20 liters per minute.
The line itself is a modular, 40 x 20-foot system composed of six machines and nine robotic components. It handles the entire production workflow, from initial card loading and inspection to final product filling and packaging.
A video demonstration showcased the system's seamless operation. Bottles are automatically unloaded, washed with oxygenated water, and then transferred by robots through the various processing stages. These include decanting, inoculation, media addition and a critical nitrogen flush to protect the sensitive cell and gene therapy products.
Throughout the process, in-line monitoring via cameras, sensors, and flow meters ensures tight quality control. All functions are managed through a central human-machine interface, allowing operators to adjust parameters as needed. Bausch stressed the importance of this adjustability, as well as the comprehensive documentation provided, to meet regulatory requirements and customer expectations.
Beyond the technical details, Bausch highlighted the critical role of effective supplier relationships and project management. He noted that providing a detailed user requirement specification upfront and conducting thorough factory and site acceptance testing are essential for a successful automation implementation.
Regulated material storage company embraces digital transformation and AI
Steffens shared his organization's journey towards digital automation and the integration of artificial intelligence to drive efficiency and compliance.
GXP-Storage, a company with over 20 years of experience in the technology industry, has been tackling the challenge of managing multiple siloed systems, a common pain point for many organizations.
"With all these wonderful technologies, all these emerging technologies and all these faster processors and the AI technology, if you don't optimize, what happens is crazy comes at you faster," Steffens reported GXP’s CEO as saying.
To address this issue, GXP-Storage has embarked on a mission to harmonize its various systems into a single platform, aiming to streamline operations and generate significant time and cost savings. Steffens explained that by integrating functions such as inventory management, equipment maintenance, and quality assurance, the company has been able to reduce the time and effort required to manage these tasks by as much as 90%.
"Now what we can do is for our operations folks that need those inventory management functions, they have their view into the system through our operations portal, for clients who need to, you know, have visibility into their inventory or request, let's say we have some material and they're going to request that we ship it back to them," Steffens said, highlighting the benefits of the integrated system.
The harmonized approach has also proven invaluable during audits and inspections. Steffens emphasized the importance of having all records and policies in a single system, allowing auditors and inspectors to easily access the necessary information.
Steffens said is excited about the potential of AI to further enhance GXP-Storage's operations. He discussed the concept of generative AI, which he believes has the power to revolutionize how users interact with business systems, much like the way Google transformed the internet search experience. Additionally, Steffens highlighted the benefits of predictive AI, which can help the company anticipate and respond to changes in temperature readings and other critical factors, ensuring compliance and the integrity of the stored materials.